Malaria Vaccine Efficacy
In each individual the event either does or does not occur 1. Participants vaccinated with R21MM showed high titres of malaria-specific anti-Asn-Ala-Asn-Pro NANP antibodies 28 days after the third vaccination which were almost doubled with the.
Develop and license malaria vaccines with protective efficacy of at least 75 against clinical malaria for areas with ongoing malaria transmission.
Malaria vaccine efficacy. In a trial in 450 children aged 517 months the vaccine called R21 was up to 77 effective at preventing malaria over the course of one year which if. The efficacy of the RTSS vaccine was established in the Phase 3 clinical trial see 3 above. Vaccine candidate R21Matrix-M has excellent potential for large-scale manufacturing and low-cost supply.
A new vaccine R21 is still in testing. The roadmap includes the following strategic goals for malaria vaccines by 2030. Vaccine developed by scientists.
Baca Juga
A shot in the arm WHO recommends malaria vaccine for use in children While the efficacy is under 50 percent it should still save numerous lives. Children who received 4 doses of the vaccine had a significantly lower risk of developing malaria including severe malaria. Vaccine efficacy began around 56 percent after year one but was close to.
High-level vaccine efficacy of 77 in African children achieve WHO-specified efficacy goal of 75. Development of malaria vaccines with protective efficacy of at least 75 against clinical malaria suitable for administration to appropriate at-risk groups in malaria-endemic areas. The trial involved 450 children aged 5-17.
A malaria vaccine developed by scientists at the University of Oxfords Jenner Institute is being hailed as a breakthrough as early trials show efficacy of 77. Due to an unexpectedly high participant retention rate we had power to detect efficacy greater than 37. Malaria vaccine efficacy VE will reflect considerable underlying heterogeneity in individual protection and immune response to the vaccine.
A potential new malaria vaccine has shown a preliminary efficacy rate of 77 during a trial on infants in what scientists hope is a breakthrough in developing a. The WHOs target efficacy for malaria vaccines is over 75. Vaccine trialled in 450 children shows favourable safety profile and was well-tolerated.
The four-country malaria trial which started on 10 May comes on the back of published phase 2 results demonstrating up to 77 efficacy for the vaccine called R21 when assessed for one year in. Oxford malaria vaccine proves highly effective in Burkina Faso trial. For the first time a vaccine has shown high efficacy in trials preventing the disease 77 of the time among those receiving it.
This article is more than 3 months old. This is a landmark achievement. We report the safety and vaccine efficacy VE of the RTSSAS01 vaccine during 18 mo following vaccination at 11 African sites with varying malaria transmission.
Development of malaria vaccines that reduce transmission of the parasite and thereby substantially reduce the incidence of human malaria infection. A malaria vaccine has proved to be 77 effective in early trials and could be a major breakthrough against the disease says the University of. The study was powered to provide an initial point estimate of the efficacy of the malaria vaccine in either group 1 or 2 assuming that the vaccine efficacy over 6 months was greater than 50.
With most infectious diseases VE compares the frequency of a specified event in the vaccinated population to that in the unvaccinated population. At 1 year vaccine efficacy remained high at 77 67-84 in group 1. A novel vaccine that uses a weakened form of a malaria parasite has produced an effective immune response in humans without any adverse side.
Despite international control efforts the malaria burden has remained constant in recent yearsThere is a strong need for a highly efficacious vaccine for malaria control and elimination which is further emphasised by increasing reports of antimalarial drug resistance and insecticide resistance and the impacts of COVID-19. 6537 infants aged 6-12 wk and 8923 children aged 5-17 mo were randomized to receive three doses of RTSSAS01 or comparator vaccine. Theres hope that endorsing a malaria vaccine now will jumpstart progress yet again.
Until now this level has never been reached. It is the first vaccine to meet the World Health Organizations WHO Malaria Vaccine Technology Roadmap goal of a vaccine with at least 75 efficacy. Develop malaria vaccines that reduce transmission and human malaria infection enabling elimination in multiple settings through.
This malaria vaccine is being used in parts of Africa to try and prevent disease but its efficacy is low. The larger phase 3 trial of the RTSSAS01 vaccine showed efficacy estimates of 283 95 CI 233 to 329 against all malaria episodes over a median of 4 years of follow-up in the group that. By James Beeson and Gaoqian Feng.
The researchers report a vaccine efficacy of 77 in the higher-dose adjuvant group and 71 in the lower dose adjuvant group over 12 months of follow-up with no serious adverse events related to. Vaccine efficacy was 74 95 CI 63-82 in group 1 and 77 67-84 in group 2 at 6 months.
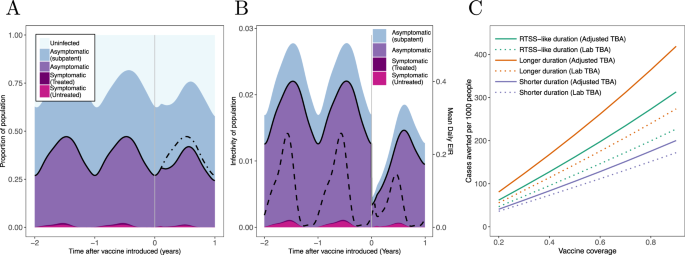
Predicting The Public Health Impact Of A Malaria Transmission Blocking Vaccine Nature Communications
Predicting The Public Health Impact Of A Malaria Transmission Blocking Vaccine Nature Communications
Visualabstract Novel Malaria Vaccine Candidate R21 Mm Is Safe And Highly Protective Against Malaria Transmission In African Children 2 Minute Medicine
Two Chemoattenuated Pfspz Malaria Vaccines Provide Strong And Lasting Protection
Vaccines Free Full Text Progress In The Development Of Subunit Vaccines Against Malaria Html
Efficacy Of A Low Dose Candidate Malaria Vaccine R21 In Adjuvant Matrix M With Seasonal Administration To Children In Burkina Faso A Randomised Controlled Trial The Lancet
Image Result For Malaria Parasite Malaria Parasite Malaria Malaria Life Cycle
Vaccines Free Full Text Pre Erythrocytic Vaccines Against Malaria Html
Why Is It Difficult To Develop A Malaria Vaccine Woidmo
Immunofocusing Humoral Immunity Potentiates The Functional Efficacy Of The Anapn1 Malaria Transmission Blocking Vaccine Antigen Npj Vaccines
Reduced Blood Stage Malaria Growth And Immune Correlates In Humans Following Rh5 Vaccination Sciencedirect
Predicting The Public Health Impact Of A Malaria Transmission Blocking Vaccine Nature Communications
Pin On Homeschool Unschool Ideas
Building A Better Malaria Vaccine
Supplemental Materials For Public Health Impact And Cost Effectiveness Of The Rts S As01 Malaria Vaccine A Systematic Comparison Of Predictions From Four Mathematical Models The Lancet
Efficacy Of A Low Dose Candidate Malaria Vaccine R21 In Adjuvant Matrix M With Seasonal Administration To Children In Burkina Faso A Randomised Controlled Trial The Lancet